Moderna mRNA approved for RSV in seniors who don't need it, against the advice of the Health Department's own committee
Same product put babies in hospital, one in the ICU, during trials in the US that were halted on safety grounds
The Therapeutic Goods Administration has approved Moderna’s mRNA product for respiratory syncytial virus gene-vaccines in seniors over age 60, despite warnings from the Advisory Committee on Vaccines that it would be of little benefit.
RSV is a mild and common respiratory virus which the Health Department said is best treated by rest and plenty of fluids.
It is not known to cause serious problems in the elderly despite claims to the contrary by drug companies.
There were only 82 recorded hospital deaths in adults aged over 65 from RSV in the decade from 1 January 2006 to 31 December 2015 across Australia, according to the Medical Journal of Australia. That is 8.2 per year - less than the risk of shark attack.
Despite this, Moderna has produced an mRNA injection called mRESVIA to “protect” adults over age 60 from RSV using the exact same repurposed gene-therapy platform used for the controversial covid products, the TGA said in its quality evaluation summary.
The covid mRNA products led to more reports of injury and death than all other vaccines in history combined, causing doctors worldwide to call for its suspension on safety grounds.
In May 2024, the Advisory Committee on Vaccines (ACV) told the TGA that the risk-benefit balance for mRESVIA in adults over age 60 was “not favourable” for a single-dose regimen. Protection waned after six months, making it useless for a second RSV season.
The ACV also said the formulation used in the trial - including the active ingredient - had been changed for the product supplied to Australians.
“The mRNA used in the drug product which was utilised during the pivotal study (RNA-100-AR01) is different to the mRNA (RNA-100-AR02) used in the drug product to be made available commercially,” the TGA’s Auspar report said.
In biologics manufacture, the way a product is made is highly specific, delicate and crucial for safety. Any little manufacturing change must go through additional trials because even the slightest tweak can alter the way it behaves.
The exact same bait-and-switch was used by Pfizer for its covid gene-vaccine (which used Moderna’s mRNA platform), with disastrous results.
Pfizer used a sterile manufacturing process that replicated RNA to make the clinical test product, while the commercial product for the public was cooked up in large vats of antibiotic resistant e. coli bacteria.
This cheap mass-production process contaminated both Pfizer and Moderna’s gene-vaccines with bits of bacterial DNA, as revealed by Dr David Speicher.
The TGA’s delegate, who makes the final decision on whether to register a vaccine or not, firmly rejected mRESVIA, saying Moderna’s trials could not show whether or not their product would work.
“Efficacy against hospitalisation associated with RSV disease could not be evaluated due to the lack of cases. There were only 2 people hospitalised in Study P301, which is less than would be expected based on the literature. This questions whether the participants in Study P301 are representative of the most at-risk populations … the Delegate was not satisfied that the efficacy of the vaccine for the purposes for which it is to be used had been satisfactorily established.”
Moderna complained and lodged a legal objection under Section 60 of the Therapeutic Goods Act 1989, demanding a review of the decision, which it got.
Moderna submitted some “fresh evidence” in the form of a couple of memos and a favourable opinion from a US CDC committee from June 2024 - before Robert F Kennedy Jr arrived as head of the Department of Health and Human Services to clear out the corruption.
The matter was then referred to a different TGA delegate who waived it through - by simply admitting there was no proof the drug would reduce hospitalisations.
“As such, no claim is made in the Australian Product Information that the product can prevent severe disease or hospitalisations,” the TGA’s Auspar report says.
Oh, that’s fine then - right?
The Therapeutic Goods Administration (TGA) approved mRESVIA (mRNA-1345) on March 28 for the prevention of lower respiratory tract disease caused by RSV for adults aged 60 and older - despite the vaccine’s effectiveness only hovering around 50 to 60 percent then quickly waning.
They put it on the Black Triangle Scheme for five years as studies are still ongoing. The Black Triangle Scheme is supposed to remind people to report any possible side effects so that the TGA can build the safety profile of a new drug, as it is not yet fully known. Most people are completely unaware of this.
Disregarded safety issues
The TGA’s analysis of the mRESVIA product continued to ignore the safety controversy surrounding the mRNA platform.
Hundreds of high-profile medical experts worldwide have spoken out against injecting Moderna’s repurposed gene-therapy technology on safety grounds. It is inherently dangerous because it goes everywhere in the body, you cannot control the dose, and there’s no off-switch.
The mRNA causes cells within your body to express a non-human protein. Your immune system has no choice but to attack your own cells to destroy it.
The immune system may then go on to attack similar cells in the body, causing auto-immune diseases in some people.
Newcastle University Immunology Professor Emeritus Robert Clancy explained this danger of “molecular mimicry” to evolutionary biologist Bret Weinstein on the DarkHorse podcast.
The lipid nanoparticle carries the mRNA everywhere in the human body including the brain and organs, and does not stay in the arm as we were told, Professor Clancy said. This potentially causes inflammation everywhere it goes, including the heart where it can cause myocarditis.
Further, a modification to replace the uridine nucleoside with N1-methyl-pseudouridine causes the mRNA to remain active in the body long after natural mRNA would be degraded.
Yale University researchers have found people still manufacturing vaccinal spike protein in their bodies up to two years post-jab as Alex Berenson reports, while a peer-reviewed study from Italian researchers in 2023 found vaccinal spikes expressed in 50 percent of subjects between 69 and 187 days post-shot.
In approving Moderna’s mRNA RSV product, the TGA ignored all of this, and said: “There were no significant issues identified to indicate the product should not be registered on the basis of quality, nor were any potential safety-related issues identified arising from the quality of the product.”
Same product halted in infant trials
The same product was halted in pediatric trials in the US after putting several children in hospital, as CHD reports.
Moderna tested mRNA-1345 (mRESVIA) and mRNA-1365 (a similar product) against an unknown placebo in infants aged 5 months to 24 months, but the study had to be halted after multiple children were made sicker than they would have been had they never had the shot.
At least one of them had to go to the intensive care unit.
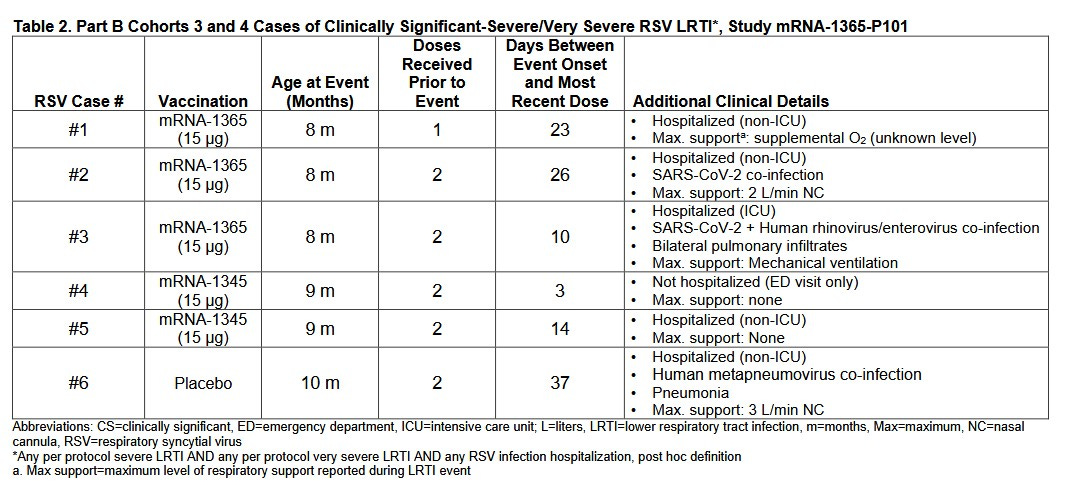
Vaccine-enhanced respiratory disease is a well-known problem when drug companies have tried to immunise infants against RSV, the US Food and Drug Administration (FDA) said in its briefing document.
“In the mid-1960s, multiple published reports described an association between FI-RSV vaccines and VAERD (Fulginiti, 1969; Kapikian, 1969; Kim, 1969). In one study of a FI-RSV vaccine in infants, 80% of vaccine recipients required hospitalization for severe RSV-LRTD upon natural RSV infection, including two children who died at the ages of 14 months and 16 months of age; in comparison, no deaths occurred and 5% of participants required hospitalization in the control group.”
RSV is more severe in infants than in adults - but it was never considered a big problem until vaccine makers had new products to sell.
It wasn’t even a nationally notifiable illness until then-Health Minister Greg Hunt made it one in 2021.
Now it’s on the notification list there are thousands of cases counted every year.
That creates a new and unmet medical need. Look how many there are. From 0 to thousands overnight.

Nurse practitioner Dr John Campbell, who trains emergency department nurses for Britain’s NHS and has spent an entire lifetime career working in hospitals said he is flummoxed as to why we need to be giving an RSV vaccine to older people for a disease that doesn’t appear to be a problem.
“I’ve worked with older people with chest infections since I was 18 on and off really, I’ve never seen an older person admitted with respiratory syncytial virus pneumonia,” he said.
“We get people in with respiratory sepsis all the time. Sometimes it’s viral but most commonly the sepsis is bacterial.”
Dr Campbell noted that the British Government had a 10-year-deal with Moderna to make millions of doses of gene-vaccines each year.
Likewise in Australia the mRNA factories have popped up in every state, tying in every major university, creating jobs that state governments and the bureaucracies want to support.
This creates a powerful and perverse incentive for governments to prevent any potential mRNA failure which may bring some unwelcome financial implications.
Imagine the pressure on regulators to simply give the desired approvals.
GOLD RUSH: Australia spends millions on mRNA factories all over a nation that can't even produce IV drip bags
Australia is hurtling into an expensive mRNA industry, squandering millions on boutique research while short-changing a country that suffers nearly 10,000 suicide attempts per month and can’t get IV bags.
There is a lot more to write about the RSV scam in Australia, particularly on how the media once again has behaved like cheerleaders and unpaid advertisers, however that will have to wait for another day.
Visit me on Twitter (X) here at LettersFromOz.
Please do share this story everywhere so people know what is going on.
SNAKE OIL: How to sell a new RSV vaccine to people who don't need it
Australians are more likely to be attacked by a shark than they are to die of Respiratory Syncytial Virus (RSV).







The more jabs you give the less drip bags you need.
This is an excellent summary of how the mRNA scam works and an article that is shareable. Thank you.