SNAKE OIL: How to sell a new RSV vaccine to people who don't need it
Don't mention the Black Triangle and pay community groups who can disguise Big Pharma messaging as expert comment for "news"
Australians are more likely to be attacked by a shark than they are to die of Respiratory Syncytial Virus (RSV).
But last month the Therapeutic Goods Administration (TGA) approved a new RSV vaccine for Australians aged over 60 - and British corporation GSK wants it put on the National Immunisation Program so your taxes can pay for it, the ABC reports.
This one product has so far earnt £1.2 billion ($2.3 billion) in global sales for GSK, contributing to total 2023 sales of £30.3 billion ($58.45 billion), but they want more.
RSV is a mild and common respiratory virus which the Health Department said is best treated by rest and plenty of fluids.
It’s not like rabies or tetanus where you’ll die without a vaccine.
There were only 82 recorded hospital deaths in adults aged over 65 from RSV in the decade from 1 January 2006 to 31 December 2015 across Australia, according to the Medical Journal of Australia.
That is 8.2 per year.
That is less than the number of shark attacks in 2022 (nine), or in 2021 (24) or in 2020 (23).
If you are under 65 the rate of RSV death is even lower, at 56 in the decade. That is 5.6 per year.
No matter what age you are, you are more likely to be attacked by a shark in Australia than you are to die of RSV.
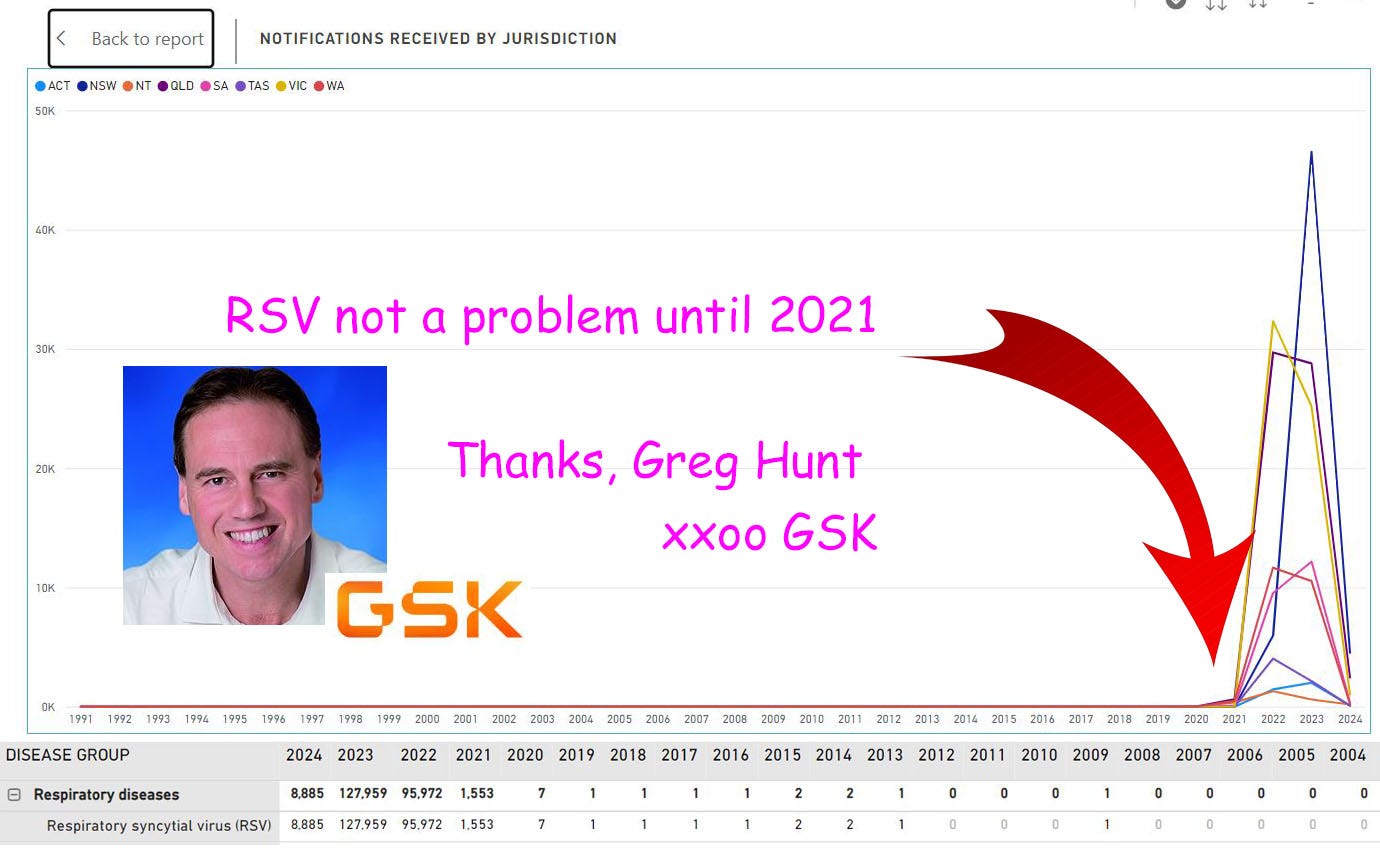
RSV wasn’t even a nationally notifiable illness until then-Health Minister Greg Hunt made it one in 2021.
Now it’s on the notification list there are thousands of cases counted every year. Shazaam! A new and unmet medical need. Look how many there are.
From 0 to thousands overnight.
On January 17, GSK announced the TGA had approved its RSV vaccine Arexvy for Australians over age 60.
It is worth noting that the GSK Australia has a good working relationship with the TGA.
GSK Australia’s head of regulatory affairs Mark McDonald worked for the TGA for 21 years, according to LinkedIn.
Dr McDonald worked in the Prescription Medicines Authorisation Branch of the Health Products Regulation Group in 2019, presenting with former TGA head Professor John Skerritt on medicines scheduling, and writing about the fast-track pathways to drug approvals.
GSK is all for fast-tracked drug approvals, as it made clear in its submission to the TGA on creating a provisional approval pathway.
Letters From Australia makes no suggestion of any impropriety.
Arexvy is on the black triangle scheme for the next five years, the TGA says.
That means it is a new medication, more risky than medications not on the black triangle scheme - and therefore subject to increased reporting requirements.
The black triangle is there to alert you, your doctor and your pharmacist to report any potential injury to the TGA, even if they are not listed on the consumer information sheet.
Regulators don’t yet know what all the side effects might be, so you are the one helping to build the safety profile of the drug by reporting your injury.
This was true for the covid vaccines which were only ever provisionally registered - but it wasn’t publicised, so thousands of deaths and injuries were never reported.
It is unclear whether the RSV vaccine is provisionally registered (meaning testing is not completed to the standard of full registration) or whether it is fully registered but also on the black triangle scheme. The TGA does not state this clearly in its decision summary.
GSK’s press release does not mention that Arexvy is on the black triangle scheme, even though the TGA said it encourages the black triangle to be used in promotional materials.
Encouragement is not enough for a company that in 2012 was convicted of health care fraud and given a US$3 billion fine, the largest in US history (to date) for the unlawful promotion of prescription drugs and failure to report safety data.
Instead of mentioning the black triangle, GSK’s press release said Arexvy was approved in the UK (also on the black triangle scheme), European Union, the US (where former FDA chief scientist Jesse Goodman sits on the GSK board, where Arexvy was fast-tracked and where Phase III trials are still ongoing for multiple doses), Canada (where it’s approved but not yet NACI reviewed) and Japan (where it cited its own press release as the source).
Letters From Australia asked GSK’s PR firm Cube why GSK’s press release does not mention the black triangle scheme. Cube said GSK was “comfortable with the accuracy of the information”.
Arexvy is not without risks.
According to the US FDA, in a study of 2500 over-60s, two participants developed a rare form of brain and spinal cord inflammation called acute disseminated encephalomyelitis (ADEM) after receiving Arexvy and the flu vaccine. One of them died.
In another two studies Guillaine-Barre syndrome and atrial fibrillation surfaced. Atrial fibrillation causes your heart to beat fast and irregularly, increasing the risk of stroke.
“The FDA is requiring the company to conduct a postmarketing study to assess the signals of serious risks for Guillain-Barré syndrome and ADEM. In addition, although not an FDA requirement, the company has committed to assess atrial fibrillation in the postmarketing study,” the FDA wrote.
MEDIA SELLING YOU DRUGS INSTEAD OF NEWS
The media has advertised the RSV vaccine without mentioning it is on the black triangle scheme or that serious side-effects are still being investigated.
Almost every expert quoted is funded in some way by GSK or GSK’s PR firm Cube.
Here’s a collection of Australia’s drug ads (sorry, I meant news stories).
Australian Associated Press - wire service
Tara Cosoleto at wire service AAP (reprinted in The Guardian, SBS News and New Daily): no mention of black triangles. Her story quotes Brisbane GP Anita Sharma who spoke at the GSK Sponsor Syposium at a medical conference in Sydney last year, to promote adult vaccination.
She also quotes Catherine Hughes from Immunisation Foundation of Australia (IFA), featured in the GSK press release.
IFA is a non-profit whose revenue mostly comes from selling programs including “RSV and Me” which promotes the RSV vaccine.
GSK donated $75,000 to the IFA in 2021, the GSK website says.
ABC News
Daniel Keane and Olivia Mason at the ABC. No mention of black triangles. Their story quotes Professor Robert Booy from the GSK press release.
Professor Booy frequently speaks in GSK press releases such as here, here and here, promoting the products and advocating for their inclusion on the taxpayer-funded National Immunisation Program.
Professor Booy receives funding from GSK along with Merck, Novartis, Pfizer, Roche and Sanofi Pasteur for sponsored research, travel to present at conferences or consultancy work; all funding received is directed to research accounts at The Children's Hospital at Westmead, according to the conflict-of-interest statement on a 2016 paper he co-authored here.
The ABC failed to mention this.
RACGP medical news site newsGP
Michelle Wisbey at the RACGP’s news site for doctors, newsGP - no mention of black triangles, even though GPs are the frontline workers who need to be aware of this.
Ms Wisbey quotes Immunisation Coalition Chair Dr Rod Pearce.
GSK paid the Immunisation Coalition $42,000 in 2021, according to the GSK charitable grants report.
The Immunisation Coalition also notes that it receives funding from vaccine manufacturers including GSK and Pfizer, in its 2023 annual report.
Professor Robert Booy, from the GSK press release, quoted in the ABC and Nine stories, is also on the Immunisation Coalition board.
Ms Wisbey also quotes Flinders University Associate Professor John Litt who has received funds for sitting on the advisory boards of GSK and Seqirus, Medicine Today reports. Flinders University is a listed sponsor of the Immunisation Coalition, alongside GSK.
So Ms Wisbey’s story quotes multiple experts that all have ties to GSK.
Nine News - webpage
Yashee Sharma at Nine News who, again, quoted Professor Robert Booy, the company man. No mention of black triangles.
Nine News TV, Sydney Morning Herald and Nine Newspapers
Nine News Eliza Edwards created a TV report republished by the Sydney Morning Herald (now owned by Nine). Backup on YouTube here. No black triangle mention.
Professor Robert Booy, from the GSK press release, is interviewed.
The story hangs on the human experience of Kate Vines, 65, from Bowral who describes how sick she was with RSV.
But Kate Vines is also connected to GSK. She is the founder, together with her husband Richard, of Rare Cancers Australia, an advocacy group for people living with rare cancers.
In 2022, GSK donated $50,000 to Rare Cancers Australia, according to the GSK website and $15,000 in 2021.
In 2016, GSK donated to Rare Cancers Australia according to Sydney University report Pharmaceutical industry funding of health consumer groups in Australia (January 2013 to December 2016), published in 2019.
Cube, the PR firm representing GSK, donates money to Rare Cancers Australia every year, raising thousands of dollars for the charity in the Mount Kosciusko challenge, such as here, and holding masquerade balls.
Rare Cancers Australia is also funded by Medicines Australia, the lobby group for big pharmaceutical firms of which GSK is a Class 1 member.
Medicines Australia donates money to Rare Cancers Australia through the Mount Kosciusko challenge along with PR firm Cube.
Letters From Australia suggests no impropriety but notes that Rare Cancers Australia is deeply connected to Big Pharma corporations and GSK both directly and through Medicines Australia and Cube.
Nine News goes on to interview Mark Brooke of Lung Foundation Australia who says:
“The Australian Government will hopefully add this vaccination to the National Immunisation Program so it becomes free for all Australians over the age of 60.”
Amazingly that is also what GSK wants, and not just for Arexvy. Last year they launched a report at Parliament House, Canberra, asking for more adult vaccines to go on the national immunisation program so your taxes can buy them in bulk, fast-tracked without delay. Doctors will then offer them “for free” to the over-60s who otherwise wouldn’t bother paying the $200 to $300 per dose reported by Nine for the RSV vaccine.
GSK, which makes Ventolin, is a corporate partner of the Lung Foundation, according to its 2022 impact report.
GSK donated $25,000 to the Lung Foundation in 2022, $52,800 in 2021 and $15,000 in 2020, the GSK website says
Vaccines on the National Immunisation Program are not free, they’re paid by your taxes, guaranteeing easy revenue for the drug company who doesn’t have to convince consumers to buy the product.
SBS News
SBS News podcast presented by Ciara Hain does not mention black triangles. She quotes Catherine Hughes from Immunisation Foundation of Australia, from the GSK press release.
Kate Vines gives the same interview as for Nine, describing how sick she was.
Then she says:
What I'd like to do is really encourage the pharmaceutical company and the government to work together to quickly have this vaccine listed on the national immunisation program.
Almost exactly what Mark Brooke of the Lung Foundation told Nine News.
It’s GSK’s key message, delivered by different people at different organisations that both get GSK funds, keeping it fresh and seemingly independent.
The Conversation
Professor Allen Cheng wrote about the RSV vaccine in The Conversation, calling for an additional RSV vaccine for young children. Again, no mention of the black triangle.
Professor Cheng was on the Australian Technical Advisory Group on Immunisation in 2020 which listed a conflict of interest disclosure due to his work for Alfred Health, which received payments from GSK.
WHAT YOU CAN DO ABOUT IT
We deserve skeptical news coverage independent of corporate funding.
A good way to encourage this is to calmly, politely and precisely complain to the outlet (eg: AAP here, SBS here or ABC here), or tip off Media Watch and the Australian Press Council (for newspapers, magazines and associated websites).
Complaining to news outlets alerts them to the problem and gets them to raise their standards - for which we’ll all feel a lot better.
Note: Please do share this story everywhere you can, so people can read my work if you think it’s worthwhile, as it really helps, especially in the age of censorship.
Updates: 18/02/24 - clarified par to say: in Canada it’s approved but not yet NACI reviewed, clarified the $200-$300 per dose is for RSV as reported by Nine/SMH, added backup YouTube for Nine story, also added the “please share” note and corrected a typo. 22/02/24 - corrected grammar (repeated word error 6th last par)









When you chew up taxpayer money on medications people don't really need, you are taking funds away from life-saving programs that people really depend on, and impoverishing us all. This is a point i forgot to make in the story - but you know it's true. There's only so much in taxes to go round.
Rare Cancers Australia is a charity that works closely with Big Pharma.
Medicines Australia and Rare Cancers Australia together launched a new Medicines Access Portal in 2022 to give doctors access to information on pharmaceutical drugs “not available to patients through normal pathways”.
https://www.medicinesaustralia.com.au/media-release/map-offers-new-online-pathway-for-accessing-cancer-treatments/
Other Medicines Australia Class 1 members also donated to Rare Cancers Australia, including Amgen, Bayer, Janssen, MSD (Merck), according to the Sydney University report on Big Pharma funding community organisations.
https://ses.library.usyd.edu.au/handle/2123/20224